Abstract
Background: Gilteritinib (formerly ASP2215), a highly selective fms-like tyrosine kinase 3 (FLT3)/AXL inhibitor, demonstrated strong antileukemic activity at doses ≥80 mg/day in a FLT3 mutation-positive (FLT3mut+) R/R AML patient population enrolled in the CHRYSALIS Phase 1/2 study (NCT02014558). In this exploratory analysis, we analyzed the impact of minimal residual disease (MRD), FLT3 allelic ratio, and FLT3 mutation status on overall survival (OS) in FLT3mut+R/R AML patients from the CHRYSALIS study.
Methods: Minimal residual disease was assessed by next-generation sequencing (NGS) using an Illumina® sequencing platform that quantified FLT3 -ITD and total FLT3 alleles in a subgroup of FLT3mut+ patients with internal tandem duplication (ITD) mutations (central laboratory-derived) who were enrolled in the 120 mg/day or 200 mg/day gilteritinib dose cohorts and had bone marrow samples available at baseline and ≥1 post-baseline time point. The ITD signal ratio was the FLT3-ITD to total FLT3 ratio. An ITD signal ratio of ≤10−4 defined MRD-negative status. For analyses of FLT3 allelic ratio and mutation status, a capture-based NGS assay that included all exons of FLT3 was used. Sample DNA was used to generate whole genome libraries which were hybridized with a custom probe to capture target fragments that were then sequenced on an Illumina® MiSeq platform. FLT3 mutation status was assessed at baseline and at relapse (investigator-assessed or central laboratory-derived relapse).
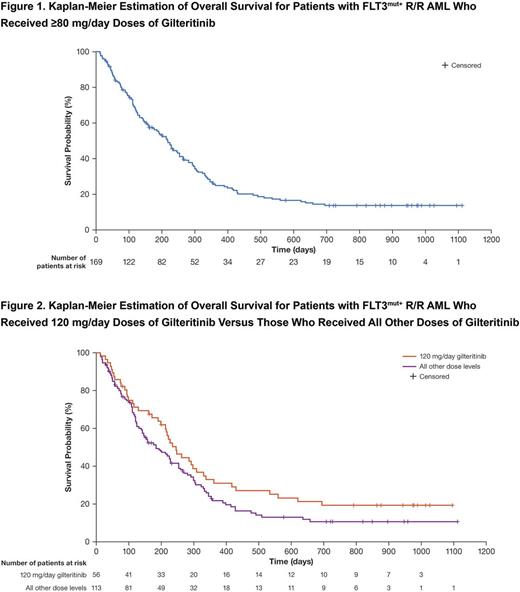
Results: Eighty FLT3-ITDmut+ patients enrolled in the 120 mg/day or 200 mg/day gilteritinib dose cohorts were analyzed for MRD. Thirteen of the 80 patients achieved MRD-negative status at any post-baseline time point. Patients who were MRD-negative (n=13; 6 achieved complete remission [CR] and 5 achieved CR with incomplete hematologic recovery) had longer median OS (417 days) than those who were MRD-positive (213 days; P=.002). There were several long-term survivors among patients who received ≥80 mg/day dose of gilteritinib (Figure 1). Of the 9 patients still remaining in the study, 5 who were included in the MRD analysis subgroup were all MRD-negative. Six of the 9 patients, including 2 of the 5 MRD-negative patients, underwent on-study transplantation. FLT3 allelic ratio was available at baseline from 6 of the 9 patients remaining on study. The median baseline allelic ratio was 61.5% in these 6 patients, which was similar to that for all 157 FLT3-ITDmut+ patients with available variant allele frequencies (50.4%).
Analysis of FLT3 mutation status in 241 patients showed that of the 35 patients with ≥1 FLT3 mutation detected at baseline and who had a relapse sample available for analysis, 27 had a relapse sample with ≥1 FLT3 mutation detected at relapse. Of these 35 patients, 15 received a starting dose of ≥200 mg/day gilteritinib and 20 received a starting dose of <200 mg/day. We detected the gatekeeper F691L mutation in relapse samples, but not in baseline samples, from 4 patients who had received <200 mg/day starting doses. In 2 of these 4 patients, 2 different DNA mutations were detected that both led to a F691L mutation, possibly suggesting the presence of multiple resistant clones at relapse. Although none of the patients who received ≥200 mg/day starting doses acquired F691L mutations, patients who received 120 mg/day doses had the longest OS of all dose groups (Figure 2). In one patient who did not undergo on-study transplantation and had re-enrolled in the CHRYSALIS study at a higher starting dose of gilteritinib (40 mg/day) and had samples available at both initial enrollment and re-enrollment, a F691L mutation that had not been detected at initial enrollment, was detected at the time of re-enrollment. One of the 4 patients with F691L mutations detected at relapse had achieved MRD negativity prior to relapse. Additional known FLT3 mutations, related to resistance, were not detected in relapse samples.
Conclusions: As a single agent, gilteritinib induced deep molecular responses, including MRD negativity, in heavily pre-treated FLT3-ITDmut+ patients with R/R AML. Our data suggest a potential association between MRD-negative status and longer survival duration in patients with FLT3-ITDmut+ R/R AML. Median FLT3 allelic ratio at baseline in patients remaining in the study was similar to that for all enrolled patients. Only F691L was detected as a known acquired resistance mutation in patients who had relapsed.
Levis: FujiFilm: Research Funding; Astellas Pharma Us: Consultancy, Research Funding; Daiichi Sankyo, Inc.: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Research Funding. Perl: Actinium Pharmaceuticals: Other: Scientific Advisory Board; Astellas: Consultancy; Arog Pharmaceuticals: Consultancy; Pfizer: Other: Advisory Board; Daiichi Sankyo: Consultancy; Asana Biosciences: Other: Scientific advisory board; Novartis: Other: Advisory Board; Seattle Genetics: Other: Advisory board. Altman: ASH: Other: Educational speaker; NCCN: Other: Educational speaker; Syros: Consultancy; BMS: Consultancy; Celgene: Consultancy; Astellas: Consultancy; Ceplene: Consultancy; Janssen Pharmaceuticals: Consultancy; Novartis: Consultancy. Cortes: BMS: Consultancy, Research Funding; Teva: Research Funding; ARIAD: Consultancy, Research Funding; Sun Pharma: Research Funding; Pfizer: Consultancy, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding. Smith: Astellas Pharma: Research Funding; Plexxikon Inc.: Research Funding. Jurcic: Astellas Pharma, Inc: Research Funding; Incyte: Consultancy; Genentech: Research Funding; Celgene: Research Funding; Daiichi-Sankyo: Research Funding; Syros Pharmaceuticals: Research Funding; Seattle Genetics: Consultancy, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy; Kura Oncology: Research Funding; Forma Therapeutics: Research Funding; Actinium Pharmaceuticals, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Alexion Pharmaceuticals: Consultancy; Amgen: Consultancy. Ritchie: Astellas Pharma: Other: Research funding to my institution; Celgene: Consultancy, Other: Travel, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Pfizer: Consultancy, Other: Research funding to my institution; Bristol-Myers Squibb: Other: Research funding to my institution; Novartis: Consultancy, Other: Research funding to my institution, and travel, Speakers Bureau; NS Pharma: Other: Research funding to my institution. Strickland: Daiichi Sankyo: Consultancy; Sunesis Pharamaceuticals: Consultancy, Research Funding; Tolero Pharmaceuticals: Consultancy; CTI BioPharma: Consultancy; Alexion Pharmaceuticals: Consultancy; Boehringer Ingelheim: Consultancy; Astellas Pharma: Honoraria; Baxalta: Consultancy. Hill: Ligacept, LLC: Equity Ownership; Astellas Global Pharma Development: Employment; Patent: Patents & Royalties: US7862995B2 - Issued; Patent: Patents & Royalties: WO2013163419A1 - pending. Liu: Abbvie: Equity Ownership; Astellas Pharma Global Development: Employment. Bahceci: Astellas Pharma Global Development: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal